Ivermectin–Old Drug,New Tricks?
Feb 16,2022
General description
Ivermectin is an antiinfective agent with activity against several parasitic nematodes and scabies and is the treatment of choice for onchocerciasis (river blindness). It is typically given as one or two oral doses. Ivermectin therapy has been associated with minor, self-limiting serum aminotransferase elevations and very rare instances of clinically apparent liver injury.Ivermectin is an FDA-approved broad-spectrum antiparasitic agent with demonstrated antiviral activity against a number of DNA and RNA viruses, including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Despite this promise, the antiviral activity of ivermectin has not been consistently proven in vivo. While ivermectin’s activity against SARS-CoV-2 is currently under investigation in patients, insufficient emphasis has been placed on formulation challenges. Here, we discuss challenges surrounding the use of ivermectin in the context of coronavirus disease-19 (COVID-19) and how novel formulations employing micro- and nanotechnologies may address these concerns.
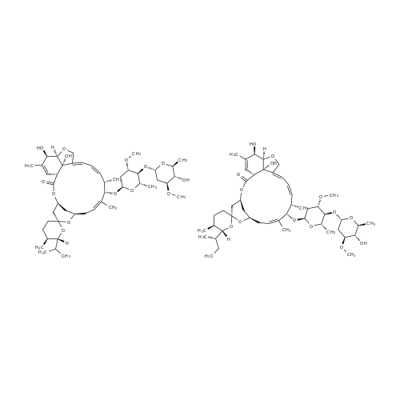
Figure the chemical structure of Ivermectin
Application and Pharmacology
A mixture of mostly avermectin H2B1a (RN 71827-03-7) with some avermectin H2B1b (RN 70209-81-3), which are macrolides from STREPTOMYCES avermitilis. It binds glutamate-gated chloride channel to cause increased permeability and hyperpolarization of nerve and muscle cells. It also interacts with other CHLORIDE CHANNELS. It is a broad spectrum antiparasitic that is active against microfilariae of ONCHOCERCA VOLVULUS but not the adult form. It hypothesize that micro- and nanotechnology-based systems for the pulmonary delivery of ivermectin may offer opportunities for accelerating the clinical re-purposing of this “enigmatic drug” in the context of SARS-CoV-2 infection, as recent advances in pharmaceutical technology and nanomaterials can be applied to the treatment of pulmonary infections. Despite the challenges faced in developing these drug delivery carriers, and uncertainty with regard to the efficacy of ivermectin, it indeed presents promising potential. In an optimistic scenario, new drug dosage forms may not only contribute to mitigate SARS-CoV-2 infection, but also be effective against other emerging viral diseases.[1]
Ivermectin (IVM) is one of the best known and most widely used antiparasitic drugs in human and veterinary medicine. From a fortuitous discovery on a Japanese golf course to a Nobel Prize, the impact of IVM on human health to date has been extraordinary. Notwithstanding the role of IVM in global food production, the Mectizan Donation Program has lifted the burden of onchocerciasis (river blindness) and, subsequently, lymphaticfilariasis (elephantiasis), from millions of people in the poorest countries in the world, and set a precedent for the role of public–private partnerships in global health. However, despite extensive research since its discovery over 35 years ago, the mode of action of IVM in parasitic species remains unclear, as are the mechanisms of resistance that allow some pathogens to survive treatment and thus the implications for current and future control strategies. Intriguingly, IVM has a diverse range of effects in many different organisms, far beyond the endoparasites and ectoparasites it was developed to control.[2]
History
Ivermectin is one of the most important drugs in veterinary and human medicine for the control of parasitic infection and was the joint focus of the 2015 Nobel Prize in Physiology or Medicine, some 35 years after its remarkable discovery. Although best described for its activity on glutamate-gated chloride channels in parasitic nematodes, understanding of its mode of action remains incomplete. In thefield of veterinary medicine, resistance to ivermectin is now widespread, but the mechanisms underlying resistance are unresolved. Here we discuss the history of this versatile drug and its use in global health.
Storage and Safety
Safety and pharmacokinetics (PK) of the antiparasitic drug ivermectin, administered in higher and/or more frequent doses than currently approved for human use, were evaluated in a double-blind, placebo-controlled, dose escalation study. Subjects (n =68) were assigned to one of four panels (3:1, ivermectin/placebo): 30 or 60 mg (three times a week) or 90 or 120 mg (single dose). The 30 mg panel (range: 347-594 µg/kg) also received a single dose with food after a 1-week washout. Safety assessments addressed both known ivermectin CNS effects and general toxicity. The primary safety endpoint was mydriasis, accurately quantitated by pupillometry. Ivermectin was generally well tolerated, with no indication of associated CNS toxicity for doses up to 10 times the highest FDA-approved dose of 200µg/kg. All dose regimens had a mydriatic effect similar to placebo. Adverse experiences were similar between ivermectin and placebo and did not increase with dose. Following single doses of 30 to 120 mg, AUC and Cmaxwere generally dose proportional, with tmax~4 hours and t1/2~18 hours. The geometric mean AUC of 30 mg ivermectin was 2.6 times higher when administered with food. Geometric mean AUC ratios (day 7/day 1) were 1.24 and 1.40 for the 30 and 60 mg doses, respectively, indicating that the accumulation of ivermectin given every fourth day is minimal. This study demonstrated that ivermectin is generally well tolerated at these higher doses and more frequent regimens.[3]
Reference
1.González Canga A., Sahagún Prieto A. M. & Diez Liébana M. J. et al., "The Pharmacokinetics and Interactions of Ivermectin in Humans—A Mini-review," The AAPS Journal, Vol.10, No.1(2008), pp.42-46.
2.Laing R., Gillan V. & Devaney E., "Ivermectin – Old Drug, New Tricks?" Trends in Parasitology, Vol.33, No.6(2017), pp.463-472.
3.Guzzo C. A., Furtek C. I. & Porras A. G. et al., "Safety, Tolerability, and Pharmacokinetics of Escalating High Doses of Ivermectin in Healthy Adult Subjects," Journal of clinical pharmacology, Vol.42, No.10(2002), pp.1122-1133.
- Related articles
- Related Qustion
- What are the functions of Ivermectin? Feb 18, 2024
Ivermectin is a remarkably potent anthelmintic and insecticide
Rhamnolipids are a class of glycolipid produced by Pseudomonas aeruginosa, amongst other organisms, frequently cited as bacterial surfactants.They have a glycosyl head group, in this case a rhamnose moiety, and a 3-(hydroxyalkanoyloxy)alkan....
Feb 15,2022Biochemical EngineeringCopper peptide (GHK-Cu), also known as liver cell growth factor, is the naturally occurring tripeptide glycyl-L-histidyl-L-lysine in a complex with copper (II), for which it has a strong affinity.....
Feb 16,2022Chemical pesticides ?Ivermectin
70288-86-7You may like
- Ivermectin
-

- $0.00 / 1G
- 2024-05-17
- CAS:70288-86-7
- Min. Order: 1G
- Purity: 99%
- Supply Ability: 20
- Ivermectin
-

- $310.00 / 1kg
- 2024-05-17
- CAS:70288-86-7
- Min. Order: 1kg
- Purity: 99.99%
- Supply Ability: 100Tons
- Ivermectin
-
- $0.00 / 1kg
- 2024-05-17
- CAS:70288-86-7
- Min. Order: 1kg
- Purity: 95.0%~102.0%
- Supply Ability: 1tons